With the increasing global concern about reducing greenhouse gas emissions and the search for cleaner and more sustainable transportation alternatives, hydrogen fuel cell cars have emerged as a promising solution. Harnessing the power of hydrogen, these vehicles offer zero-emission driving while maintaining long-range capabilities. In this blog post, we will delve into the inner workings of hydrogen fuel cell cars, exploring various subtopics to unravel the science behind this groundbreaking technology.
Understanding Hydrogen Cells:
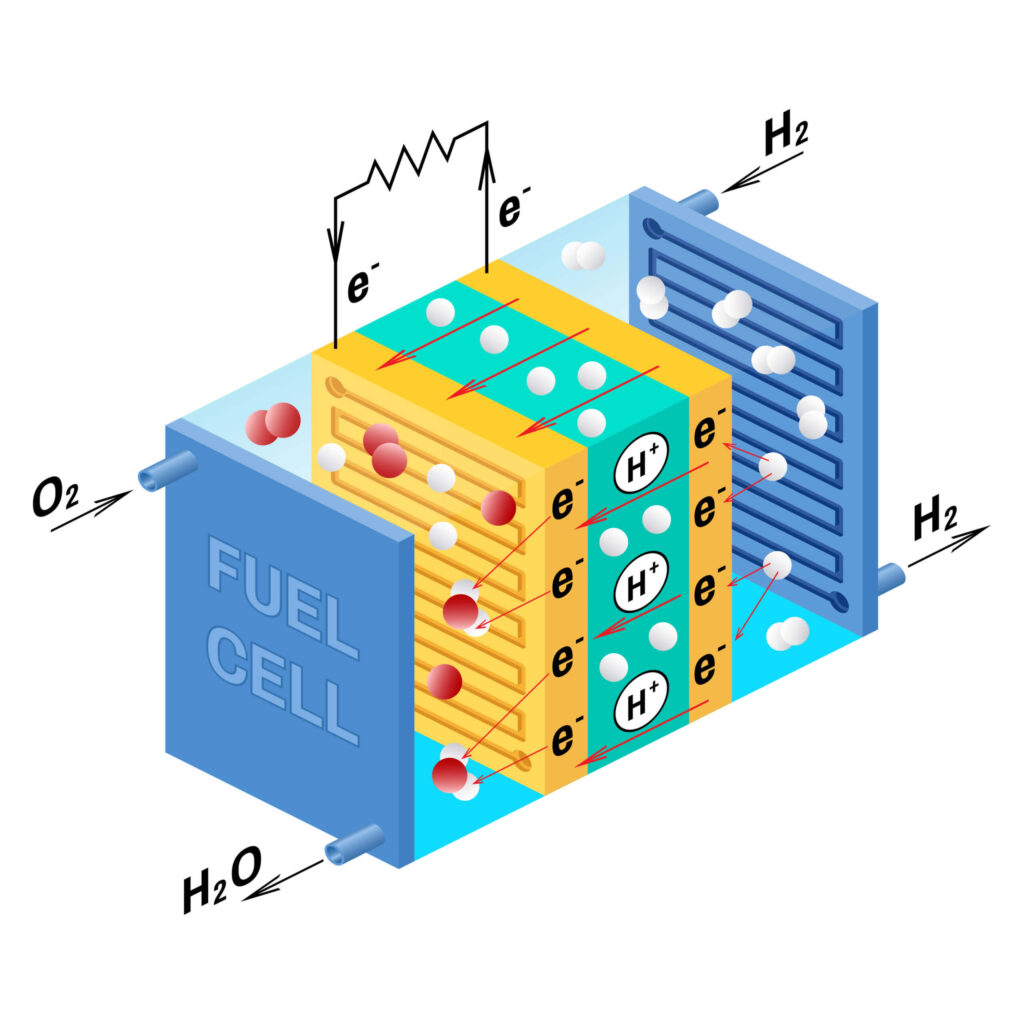
Fuel cells are electrochemical devices that convert the chemical energy of a fuel, such as hydrogen, directly into electrical energy without combustion. They consist of an electrolyte, an anode, a cathode, and catalysts. The purpose of a fuel cell is to generate electricity by facilitating a controlled reaction between the fuel and an oxidant, typically oxygen. In a hydrogen fuel cell, the electrolyte separates the anode and cathode and allows only positive ions to pass through. The anode receives hydrogen gas (H2) and splits it into protons (H+) and electrons (e-). The protons migrate through the electrolyte to the cathode, while the electrons travel through an external circuit, creating an electrical current. At the cathode, oxygen from the air reacts with the protons and electrons to form water as the only byproduct.
How are Hydrogen Cells made?
There are several methods to produce hydrogen, including steam methane reforming (SMR), electrolysis, and renewable sources such as biomass or solar-powered electrolysis. SMR involves reacting methane with steam to produce hydrogen and carbon dioxide. Electrolysis splits water molecules into hydrogen and oxygen using electricity, while renewable sources utilize sustainable energy to power the electrolysis process. Hydrogen can be stored in various forms, including compressed gas, liquid, and solid-state storage. Compressed hydrogen gas is stored in high-pressure tanks, while liquid hydrogen requires cryogenic conditions. Solid-state hydrogen storage involves metal hydrides or complex compounds capable of absorbing and releasing hydrogen under controlled conditions.

The Chemistry behind it
At the cathode, the oxygen reduction reaction occurs, where oxygen molecules (O2) combine with electrons and protons from the anode to form water (H2O). Meanwhile, at the anode, the hydrogen oxidation reaction takes place, involving the splitting of hydrogen molecules (H2) into protons and electrons. The overall cell reaction combines these reactions, generating electricity and producing water as the only byproduct. Catalysts, such as platinum, are crucial in fuel cell operation as they speed up the electrochemical reactions without being consumed in the process. Platinum catalysts are widely used due to their effectiveness, but they are expensive. Research efforts are focused on developing alternative catalysts that are more cost-effective while maintaining high efficiency. PEMFCs are a common type of fuel cell used in hydrogen-powered vehicles. They use a solid polymer membrane as the electrolyte, enabling fast proton transport and efficient operation at relatively low temperatures. PEMFCs offer rapid startup, high power density, and compact designs, making them suitable for automotive applications.
Powering the Vehicle
In a hydrogen fuel cell vehicle, the electrical energy generated by the fuel cell stack powers an electric motor, providing propulsion. Power distribution and control systems manage the flow of electricity between the fuel cell, motor, and auxiliary components. Voltage regulation and power management systems ensure efficient utilization of the generated power and enable various vehicle functions. Hydrogen fuel cell vehicles offer advantages in terms of efficiency and performance compared to internal combustion engine vehicles. Fuel cell systems can convert a higher percentage of the energy stored in hydrogen into usable electricity, resulting in higher overall efficiency. Additionally, hydrogen fuel cell cars provide instant torque, smooth acceleration, and long driving ranges, making them comparable to conventional vehicles in terms of performance.

Infrastructure and Challenges
Establishing a network of hydrogen refueling stations is essential for widely adopting hydrogen fuel cell vehicles. Currently, the infrastructure for refueling stations is limited, but efforts are underway to expand their availability. Governments and private companies are investing in the development of refueling infrastructure, including partnerships with automotive manufacturers to promote the growth of hydrogen fuel cell technology.
Despite the promising potential of hydrogen fuel cell vehicles, there are challenges to overcome. Cost reduction is a significant hurdle, primarily due to the high cost of platinum catalysts and the current limited production scale. Advancements in catalyst materials, storage technologies, and production methods are being pursued to address these challenges and make hydrogen fuel cell vehicles more cost-effective and accessible. Continued research and development, as well as increased support from governments and the automotive industry, are vital for the future prospects of this technology.

Final Thoughts
Hydrogen fuel cell cars represent an exciting leap towards a sustainable and zero-emission transportation future. By unraveling the science behind hydrogen fuel cells, understanding hydrogen production and storage methods, exploring power generation processes, and considering infrastructure challenges, we gain insight into the immense potential of this technology. With ongoing advancements, increased investments, and a focus on environmental sustainability, hydrogen fuel cell cars have the potential to revolutionize the automotive industry, providing clean and efficient transportation while preserving our planet for future generations.


